2023
2022
2021

4
Aug
Despite evidence for associations between low circulating 25(OH)D concentration and kidney disease, few clinical trials have evaluated the effect of vitamin D supplementation on kidney...

1
Jul
Observational studies suggest that low vitamin D status may be a risk factor for cancer. In a population with prediabetes and overweight/obesity that is at...
2020

19
Oct
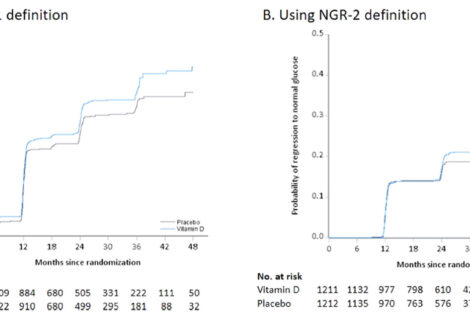
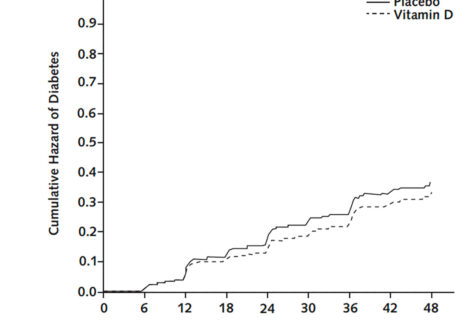
The manuscript explains that results from recent trials are congruent with a large body of evidence from observational studies indicating that vitamin D has a role...

5
Oct
The manuscript explains how postrandomization biases may influence the estimate of efficacy of supplemental vitamin D in diabetes prevention trials. In the Vitamin D and...

1
Jul
In the commentary Dr. Pittas and Dr. Balk explain that although meta-analyses increase statistical power, allowing more precise effect estimates, for them to be credible...

22
Jan
This analysis used D2d data (individuals with complete screening and baseline visit data) to calculate HGI, identify demographic and clinical variables associated with HGI, and...
2019

7
Jun
A presentation of the results took place at the annual American Diabetes Association meeting in San Francisco on Friday, June 7th. Results were published in...
2018

2
Nov
The last participant study visit was completed at the Northwestern site. This is a very exciting time for both participants and researchers alike! Since the...

3
May
Randomized clinical trials that have public health implications but no or low potential for commercial gain are predominantly funded by governmental (e.g., National Institutes of...

6
Apr
A future long-term observational follow-up study (with no pills or visits) is being considered for the purpose of collecting simple health information to learn more...

3
Mar
D2d is the largest clinical trial designed specifically to examine the causal relationship between vitamin D supplementation and the development of diabetes. D2d is also...
2017

22
Oct
The annual meeting of the D2d research team is held in Washington D.C.. On Sunday evening, Principal Investigator Dr. Pittas presents the State of D2d, which...

25
Sep
The Data and Safety Monitoring Board (DSMB) reviews the results of the interim analysis. This is a critical juncture in the conduct of any clinical...

3
Aug
The National Diabetes Education Program created the Small Steps. Big Rewards. GAME PLAN. toolkit to deliver basic type 2 diabetes prevention information to individuals at risk....

8
Feb
A patient from the New York Lenox Hospital site is the 2,423rd and last participant enrolled in D2d. The participant completed the study. Congratulations to...
2016

16
Dec
A participant from the Atlanta VA Medical Center site is the 2,382nd participant enrolled in D2d. The study’s recruitment goal is achieved. This is a...

29
Nov
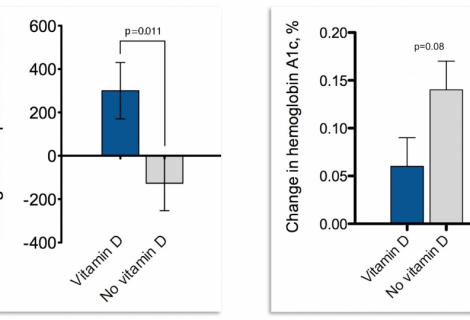
When measuring HbA1c, many clinical laboratories do not report the presence of hemoglobin variants, whereas others report them only if they interfere with HbA1c measurement....

25
Sep
The annual meeting of the D2d research team is held in Arlington, VA. On Sunday evening, principal investigator Dr. Pittas presents the State of D2d,...

27
Jun
A participant from the Pennington Biomedical Research Center site is the 2,000th participant enrolled in D2d. The participant completed the study....
2015

20
Nov
A participant from the Northwestern University site is the 1,500th participant enrolled in D2d. The participant completed the study....

8
Nov
The annual meeting of the D2d research team is held in Arlington, VA. On Sunday evening, principal investigator Dr. Pittas presents the State of D2d,...

20
Apr
A participant from the MedStar Community Clinical Research Center D2d site is the 1,000th participant enrolled in D2d. The participant completed the study. ...
2014

20
Nov
The D2d Data and Safety Monitoring Board (DSMB) meets twice a year to review the study’s progress. At this meeting, the DSMB is impressed with...

17
Nov
The annual meeting of the D2d research team is held in Crystal City, MD. On Sunday evening, principal investigator Dr. Pittas presents the State of...

15
Sep
The D2d team publishes its first manuscript related to D2d: The rationale and Design of the Vitamin D and Type 2 Diabetes (D2d) Study: A...

28
Aug
A participant at the Atlanta VA Medical Center site is the 500th participant enrolled in D2d. The participant completed the study....
2013

1
Nov
A patient at the University of Kansas site is the 1st participant enrolled in D2d. The participant is still in D2d....

21
Oct
Tufts Medical Center (where the D2d Coordinating Center is based) and NIDDK coordinate a press release to announce the official start of D2d activities....

15
Oct
D2d begins screening people at high risk for diabetes to join D2d. Screening takes place in many medical centers throughout the United States. The first...

1
Oct
The U.S. federal government enters a shutdown because neither legislation appropriating funds for fiscal year 2014 nor a continuing resolution for the interim authorization of...

19
Sep
Obtaining IRB approval from each separate collaborating clinical site has long been considered a major reason for delaying the start of activities in multi-center trials....

9
Jul
The first meeting of the D2d Research Group is held in Bethesda, MD. Investigators and coordinators from all sites and all supporting units (e.g., central laboratory,...

1
Jun
Tufts Medical Center receives the U01 cooperative agreement grant to fund and conduct D2d. The grant is awarded by NIDDK and the NIH Office of...

31
May
The inaugural meeting of the D2d Data Safety Monitoring Board (DSMB) takes place in Bethesda, MD. The core D2d team – composed of Dr. Pittas...
2012

14
Sep
The U01 grant application seeking funding for D2d undergoes a highly favorable scientific peer review by a NIH Special Emphasis Study Section, specifically convened to...

5
Jun
The D2d team submits a U01 grant application to NIH for funding to conduct D2d. Assembling a 1,140-page grant application was a Herculean effort by...

30
Mar
An External Evaluation Committee convened by NIDDK reviews the progress of the U34 planning phase. The Committee concludes that the D2d team has made excellent...
1
Mar
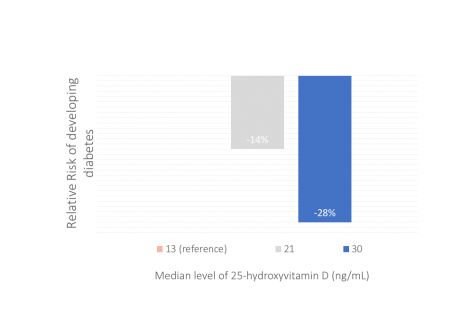
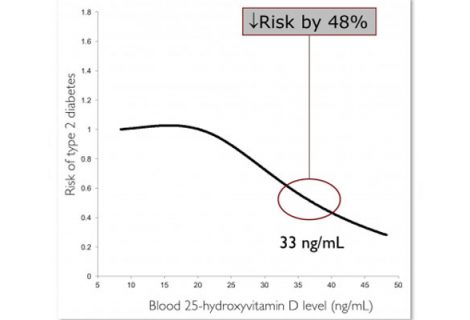
This observational study was to assess the longitudinal association between vitamin D status – assessed by blood level of 25-hydroxyvitamin D – and risk of developing diabetes among...
2011

11
Aug
The D2d logo was created in the summer of 2011. The three elements (D, 2, d) were chosen to represent the D2d study in the...
1
Aug
Results of the Vitamin D and Calcium Homeostasis for Prevention of Type 2 Diabetes (CaDDM) study support a mechanistic link between vitamin D and diabetes...

20
Jul
Dr. Pittas and colleagues are awarded a Multi-Center Clinical Study Implementation Planning Cooperating Agreement (U34) grant. The NIDDK-supported grant provides support to a core team...
2010

10
Oct
The U34 grant application undergoes a highly favorable scientific peer review by an NIH Special Emphasis Study Section, specifically convened to review the application. Over...
15
Sep
An observational study in the Nurses’ Health Study – a longitudinal cohort of women – supports an association between higher blood level of vitamin D and...

30
Jul
Dr. Pittas and colleagues submit the Multi-Center Clinical Study Implementation Planning Cooperating Agreement (U34) grant application to NIH. The goals of the U34 grant are...

1
Jul
During the spring and early summer of 2010, the D2d concept goes through additional rounds of review within NIDDK. In July of 2010, Dr. Pittas...

15
Jan
In December of 2009, Dr. Pittas submits a pre-application to NIDDK proposing the study concept of D2d. The scientific justification of D2d and the preliminary...
2008

25
Sep
Dr. Pittas and colleagues receive funding by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and the Office of Dietary Supplements (ODS)...
2007

31
Jul
Dr. Pittas and colleagues receive funding by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH)...
1
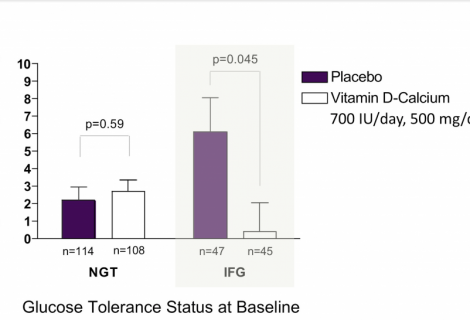
Apr
In a post-hoc analysis using data from a completed trial, vitamin D and calcium supplementation given over 3 years improved insulin resistance and fasting blood...
2006

30
Sep
Based on strong preliminary data generated by Dr. Pittas and colleagues, the Vitamin D and Calcium Homeostasis for Prevention of Type 2 Diabetes (CaDDM) study...
2002

10
Jul
The idea that vitamin D is linked to risk of developing type 2 diabetes is conceived by Dr. Pittas. In 2002, Dr. Pittas enrolled in...