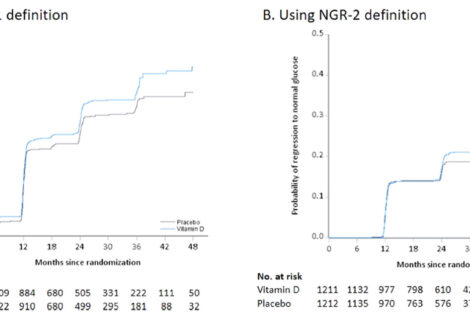
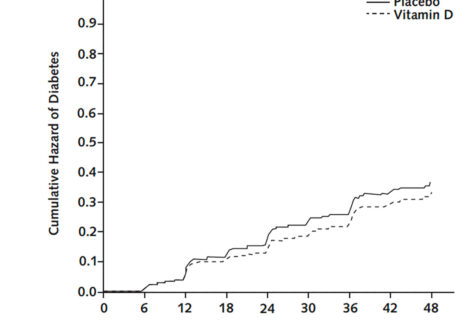
Routine use of vitamin D supplements has increased substantially in the United States. Hence, establishing the safety and tolerability of long-term use of high-dose vitamin D is critically important.
This pre-specified analysis in D2d assessed the safety and tolerability of vitamin D3 at a dose of 4000 IU per day in a population that is overweight/obese and at high risk for diabetes.
Safety events, including laboratory values (e.g., calcium) and outcomes (e.g., kidney stones), were carefully recorded during the study. During 3 years of follow-up, adverse events were less frequent in the vitamin D group compared to the placebo group. The overall frequency of protocol-specified adverse events of interest, which included nephrolithiasis, hypercalcemia, hypercalciuria, or low estimated glomerular filtration rate, was low and did not differ by group. There were no significant between-group differences in total serious adverse events.
These results provide reassuring data of the safety and tolerability of long-term use of high-dose vitamin D.
The manuscript was published in the European Journal of Clinical Nutrition.