Oct
15
2013
D2d begins screening people at high risk for diabetes to join D2d. Screening takes place in many medical centers throughout the United States. The first participant is screened on October 16, 2013 at the Florida Hospital Translational Research Institute...
Oct
01
2013
The U.S. federal government enters a shutdown because neither legislation appropriating funds for fiscal year 2014 nor a continuing resolution for the interim authorization of appropriations are enacted before the new fiscal year begins. Participant recruitment in D2d is...
Sep
19
2013
Obtaining IRB approval from each separate collaborating clinical site has long been considered a major reason for delaying the start of activities in multi-center trials. D2d becomes the first NIDDK-supported trial and only the fifth NIH-supported study to join...
Jul
09
2013
The first meeting of the D2d Research Group is held in Bethesda, MD. Investigators and coordinators from all sites and all supporting units (e.g., central laboratory, central pharmacy) plan a successful study launch.
Jun
01
2013
Tufts Medical Center receives the U01 cooperative agreement grant to fund and conduct D2d. The grant is awarded by NIDDK and the NIH Office of Dietary Supplements. Project Manager Patty Sheehan and Principal Investigator Anastassios Pittas (shown in the...
May
31
2013
The inaugural meeting of the D2d Data Safety Monitoring Board (DSMB) takes place in Bethesda, MD. The core D2d team – composed of Dr. Pittas (principal investigator), Dr. Staten (NIH project scientist), Dr. Ware (lead statistician) and Ms. Sheehan...
Sep
14
2012
The U01 grant application seeking funding for D2d undergoes a highly favorable scientific peer review by a NIH Special Emphasis Study Section, specifically convened to review the application. The review panel considers the application “outstanding” and gives a priority...
Jun
05
2012
The D2d team submits a U01 grant application to NIH for funding to conduct D2d. Assembling a 1,140-page grant application was a Herculean effort by the D2d planning team. Many scientific, logistical, financial and administrative components came together to...
Mar
30
2012
An External Evaluation Committee convened by NIDDK reviews the progress of the U34 planning phase. The Committee concludes that the D2d team has made excellent progress in designing the D2d study and in preparing for the clinical trial phase....
Mar
01
2012
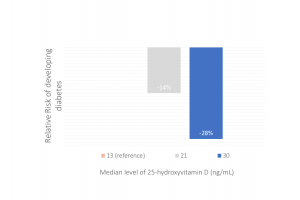
This observational study was to assess the longitudinal association between vitamin D status – assessed by blood level of 25-hydroxyvitamin D – and risk of developing diabetes among people at high risk for diabetes (i.e., with prediabetes). The case-cohort study followed participants...











 Know someone, a friend or family member, who might be interested in learning about D2d? You can easily share with others by email or on social media.
Know someone, a friend or family member, who might be interested in learning about D2d? You can easily share with others by email or on social media.